1. How do plant pathogenic fungi build specialized infection cells called appressoria?
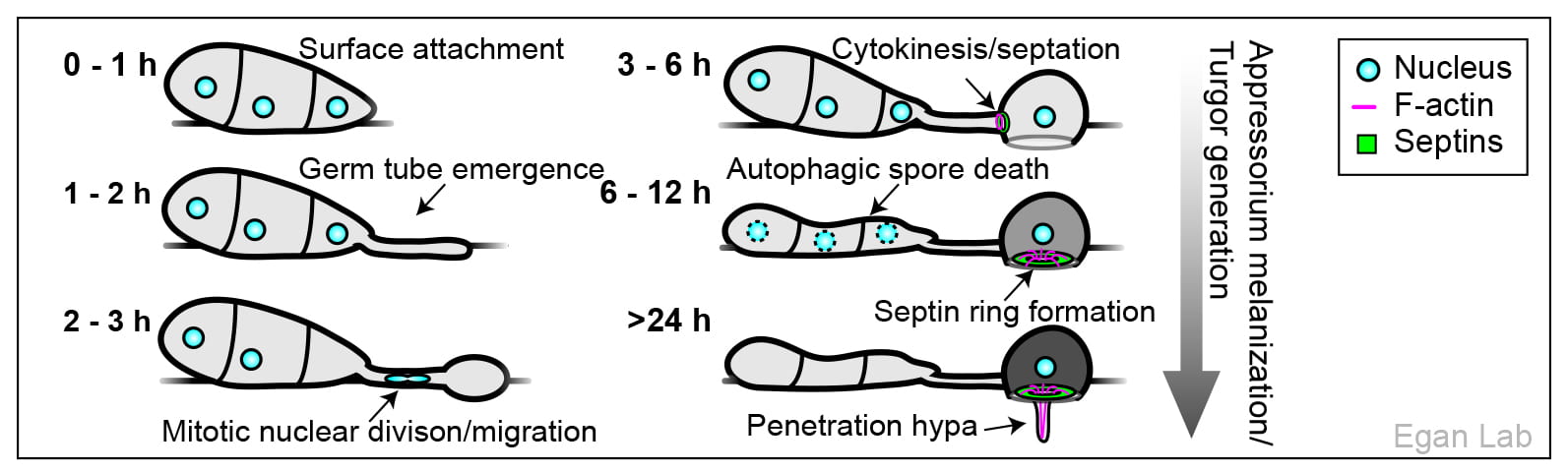
Rice blast disease, caused by the fungus Magnaporthe oryzae, destroys enough rice each year to feed 60 million people, and is a major threat to global food security. The recent emergence of a wheat-adapted population now similarly threatens wheat production world-wide. To infect plants, M. oryzae forms a specialized infection cell called an appressorium, which it uses to break into rice leaves using enormous osmotic turgor, equivalent to 40 times the pressure in a car tire. Essential for this process is the formation of a higher-order septin ring structure at the base of the appressorium, which scaffolds a donut-shaped filamentous actin network, needed for the emergence of a polarized penetration structure from the base of the appressorium. A major focus of our research is understanding how this process is controlled in space and time, with the long term goal of identifying novel control strategies for rice blast disease. The ability of M. oryzae to form appressoria containing septin rings, on glass surfaces, in under 8 hours, combined with its high genetic tractability, make this an unparalleled system for understanding appressorium morphogenesis.
2. How do highly polarized cells types deal with stress induced protein aggregation, and what are the consequences of dysfunction in these pathways?
Eukaryotes have evolved a number of strategies to manage misfolded and aggregated proteins and therein maintain protein homeostasis (proteostasis) in response to cellular stress. These include proteolytic pathways such as the ubiquitin proteasome system and restorative pathways in which damaged proteins are rescued by molecular chaperones and disaggregases. In humans, a breakdown in proteostasis is linked with diverse pathologies including neurodegenerative diseases and cancer. A cellular hallmark of these diseases is the presence of cytoplasmic and/or nuclear inclusion bodies which for many years were thought to contribute to disease establishment and progression. However, a growing body of evidence reveals that inclusion bodies form as part of a highly-orchestrated cellular protective pathway called spatial protein quality control (SPQC), in which damaged proteins are sequestered and confined to discrete quality control compartments to be processed later by the cell. The long-term goals of my research program are to determine in mechanistic detail how, where, when, and why damaged proteins are recruited to inclusion bodies and to understand the cellular consequences of dysfunction in this pathway. We are using the filamentous fungus Aspergillus nidulans as an innovative model system to understand SPQC in highly polarized cells.
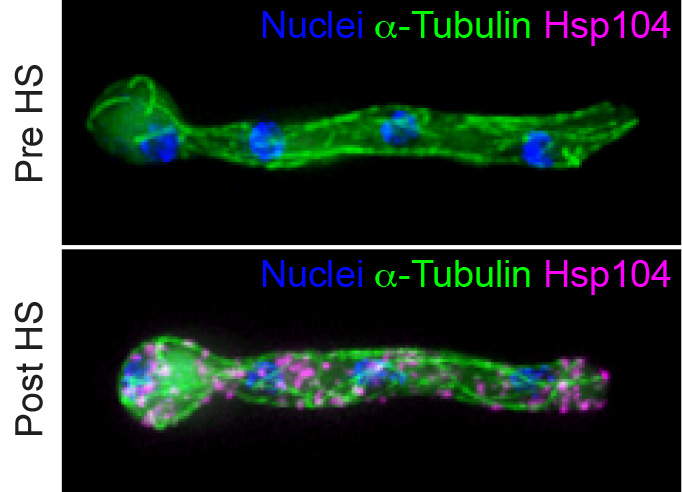
Hsp104-labeled protein aggregation in response to heat stress in Aspergillus nidulans
3. Long-distance intra-hyphal transport in fungal growth and pathogenesis
The capacity of Aspergilli to cause opportunistic infections in humans, is dependent on their ability to form highly polarized cell-types called hyphae, which grow almost exclusively by apical expansion. Importantly, the extreme polarization of Aspergillus hyphae means that their growing hyphal tips, which are first to perceive the ambient environment, are located at considerable distances from their nuclei which control morphogenetic development. The molecular and cellular mechanisms by which filamentous fungi communicate sensory information perceived at their hyphal tips to their distal multinucleated hyphal compartments to initiate adaptive responses, remain unclear. We are exploring the contribution that long-distance microtubule-based early endosome trafficking plays in tip-to-nucleus communication during pathogenesis in A. fumigatus. Additionally, we are broadly interested in understanding how microtubule-based intra-hyphal transport is controlled in filamentous fungi to drive rapid polarized cellular growth.